MBI Videos
Miquel Marin-Riera
-
 Miquel Marin-Riera
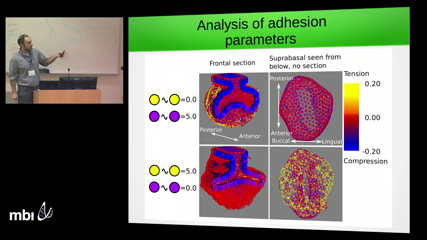
Miquel Marin-RieraWe want to understand the mechanisms that drive cell movement and tissue deformation during morphogenesis in order to predict how changes in development produce morphological variation that is relevant for evolution. We use the mammalian tooth as a model system due to the large variation it shows across the phylogenetic tree and its relatively well known development. Despite an extensive knowledge on the molecular pathways regulating the patterning and morphogenesis of the developing tooth, little is known about how individual cells move and what mechanisms are driving these movements. In order to shed light on those mechanisms, we design a mathematical model of tooth development in which cell movements are driven by compressive and tensile mechanical forces originating from tissue growth and cell-cell adhesion. The model is set to reproduce the transition between mouse molar bud (E13) and cap cap stages (E15), during which two epithelial folds protrude from the epithelial tooth germ and surround the underlying mesenchyme. When we fit the tissue specific growth rates in the model to the ones estimated from experimental data, the model correctly predicts the morphology of the tooth germ and the directionality of cell trajectories in the epithelial compartment. The model also predicts that different spatial patterns of mechanical forces arise when the adhesion strength between different cell types is varied. In order to validate the model predictions we experimentally infer the forces by means of mechanical perturbations on dissected tooth germs. We conclude that a simple model of differential growth and adhesion is able to explain the morphology, patterns of cell movement and partially the mechanical forces generated during tooth development. We argue that the addition of active cell migration might be required in order to improve the model predictions.
